Ⅰ. Introduction
Humans have been at war with numerous viruses since their birth. Many people lost their lives during the smallpox and Spanish flu epidemics in the 20th century. became ill Since 2019, the global pandemic of COVID-19 (Coronavirus disease-2019) has caused 5,763,152 deaths, and various mutations have made it difficult to cope with it.[1] Accordingly, for the safety of mankind, the development of vaccines and treatments through the study of the structure and life history of viruses is becoming very important.
There are various viruses on Earth. Most viruses invade the host cell of an organism, replicate their genetic material, and synthesize proteins to produce offspring. When these viruses enter the body, the body's immune system triggers various actions to remove them. According to the high school curriculum Life Science I, when foreign substances such as pathogens and foreign substances invade the body, macrophages present antigens on the surface through phagocytosis to form helper T cells and cells It activates cytotoxic T cells, and the activated helper T cells help activate B cells and cytotoxic T cells and differentiate themselves into memory helper T cells. In addition, cytotoxic T cells and B cells differentiate into memory T cells, memory B cells, and plasma cells, respectively, to form an immune system. Through this process, the human body forms an immune system to remove foreign substances and prepares for invasion again in the future. However, if there is a virus that uses and destroys such an immune system, it will be fatal to mankind by disrupting the immune system that operates sophisticatedly and is directly related to life. HIV (Human Immunodeficiency Virus), which is a cause of Acquired Immune Deficiency Syndrome (AIDS), corresponds to such a virus. HIV infects and destroys cells responsible for the immune system, such as helper T cells and macrophages, as hosts. Therefore, it is very important to research a treatment method because the body's immune system alone cannot cure it. Recognizing this importance, various treatments have been developed through HIV treatment research for the past 30 years. However, no cure has been found yet to completely remove HIV from the patient's body, and about 1 million people die every year from AIDS caused by HIV. Therefore, this study aims to present a treatment method that can be applied to each stage of life history by focusing on analyzing and eradicating the life history of HIV.
II. Theoretical background
1. HIV Life Cycle

The HIV life cycle is complex and can be simplified as shown in Fig. 1.[2] HIV approaches the target cell, CD4+ helper T cell, and binds gp120 on the surface of HIV to the CD4 major receptor located on the surface of the target cell, and then fuses with the target cell. In this case, CCR5 (or CXCR4), a co-receptor on the surface of the target cell, is an essential molecule in binding to the cell membrane. When fusion occurs, the genetic information of HIV is introduced into the host cell, and the RNA of HIV is reverse transcribed into DNA by the reverse transcriptase of HIV. Such proviral DNA is introduced into the nucleus of the host cell, and with the help of integrase, the genome of HIV is inserted into the genome of the host cell. Due to this, the genome of HIV is replicated together with the DNA of the host cell, and after replication, it is activated by immunostimulation, which undergoes transcription and translation through the RNA polymerase of the host cell to produce the protein of HIV.[3] New HIV generated in this way destroys the host cell and is released out of the cell, but it is still immature and non-infectious, so it goes through a maturation process. As a result, the proteins are rearranged to form a cone-shaped inner nucleus, which becomes infectious and regenerates into mature HIV.
1.1. Viral Attachment and Membrane Fusion
Since HIV, which has not invaded cells, usually has a half-life of about 20 minutes, it is very important to find and infect new host cells within a short period of time. In this process, the main receptor CD4 on the surface of the target cell and the co-receptor CCR5 (or CXCR4) play a key role, and they help the HIV invasion into the target cell. However, since the density of Env trimer on the HIV surface binding to the receptor and the density of CD4 on the target cell surface are generally low, the viral conjugation process in HIV infection is somewhat limited. Therefore, other receptors on the surface of the target cell, such as poly-glycan and lectin, temporarily conjugate with HIV, thereby increasing the density of HIV on the cell surface, thereby helping to interact with CD4 and CCR5. As a result, the likelihood of infection increases, resulting in a significant increase in HIV production.
On the surface of HIV, there is a membrane protein, a complex of gp120 constituting the head and gp41 constituting the stem. Infection occurs through the interaction of target cell CD4 with the HIV glycoprotein gp120. By inducing a conformational change in Env trimer that CD4 activates the interaction of gp120 with CCR5 or CXCR4, gp41 injects a hydrophobic fusion peptide into the host cell membrane. Thereafter, gp41 forms a helical bundle structure to fuse the membrane between HIV and the host cell and transport the HIV substance into the host cell.
1.2. Reverse Transcription
When the membrane fusion is achieved, the genetic information of HIV is injected into the cell. One of the two RNA (+) strands of HIV protected by a nucleocapsid undergoes reverse transcription to form double-stranded DNA. Reverse transcription refers to a central principle, namely, that the transcriptional steps of transcription and translation are reversed. In a typical cell, RNA polymerase binds to DNA and a transcription process takes place using one strand of DNA as a template to synthesize RNA. However, the reverse transcription process is accomplished by synthesizing DNA using RNA as a template by binding of reverse transcriptase to RNA. This is a characteristic of viruses of the Retroviridae family, mainly including HIV. After inserting the DNA generated by the reverse transcription process into the genome of the host cell, enzymes in the host cell are used to synthesize the necessary protein.
1.3. Uncoating
Before the HIV genome is injected into the nucleus of a host cell, the helical bundle structure carrying the HIV genome, that is, the capsid of HIV, must be disassembled. Since there are many enzymes that degrade RNA and DNA in nature, HIV RNA and DNA are protected through the capsid. Prior to degradation of the capsid, the capsid of HIV remains in a stable state until it can cause infection in the host cell, which is very important in the aspect of infection of HIV. However, the exact timing of the breakdown of the capsid of HIV remains a major challenge for current research.[4]
1.4. Nuclear Entry
Since HIV belonging to a lentivirus can infect nondividing terminally differentiated cells such as macrophages, the genome of HIV must be transported through an intact nuclear membrane. Also, since the PIC (Pre-Integration Complex) that helps transport the HIV genome is very large, it cannot pass through the nuclear membrane of the host cell by passive diffusion, so it is transported through active transport through the nuclear pores.
1.5. Integration
After HIV produces double-stranded DNA and transports it into the nucleus through the nuclear membrane of the host cell, its genome is inserted into the genome of the host cell through an enzyme called integrase for expression and effective infection. . Thereafter, the infected host cell usually remains infected for the remainder of the cell cycle, and the genetic information of HIV inserted during DNA replication is also replicated. Due to this, HIV not only spreads to new cells, but also causes transmission in a way that continuously replicates from one host cell and increases the number. In particular, the genome of HIV can maintain an incubation period for several years in the form of a provirus combined with the genome of a host cell in a cell that is preserved for a long time, such as a memory T cell. Therefore, even when cells are observed to determine disease progression or infection, it is not clear to determine the presence of HIV in the incubation state. It can be activated and cause symptoms.
1.6. Transcription


Fig. 2 is a schematic diagram of the HIV genome and Fig. 3 is a mature HIV virus.[5][6] HIV in Fig. 3 is in the virion state, which is the state when it is outside the host. In the host cell, the HIV provirus serves as a template used for transcription of HIV mRNA and genomic RNA by Pol II Polymerase in the host cell. The transcription of the HIV provirus is initiated by the HIV promoter, which is located at the U3 point of the 5' LTR. The expression of HIV is determined by host cell transcription factors such as NF-kB and NFAT. The RNA elongation process of early HIV is very inefficient, the transcription rate is very slow, and the transcription-promoting protein Tat (Transactivator protein Tat) is required. Tat plays a role in enhancing the reaction progress of the transcription process by binding to the R site of the 5' LTR. In this process, Tat enables an efficient synthesis reaction of the HIV transcription process, and more than 25 mRNAs are generated in three sizes through splicing, that is, the process of linking exons by removing introns from RNA. The first is a 9kb RNA that has not undergone splicing, which serves as a genomic RNA or forms a Gag or Gag-Pol precursor. The second is a 4kb RNA that has been partially spliced and encodes information of Vif, Vpr, Vpu, and Env. The third is a 2kb RNA that has completed the splicing process, and contains Tat, Rev, and Nef information.
1.7. Translation
Among the regulatory genes of HIV, Tat plays a big role in promoting the transcription process of HIV genetic information and RNA elongation process, and Rev regulates the process of transporting 9kb and 4kb RNA into the cytoplasm of the host cell. Synthesis of Tat and Rev produces mRNA that has not undergone splicing, and the RNA expresses Gag and Gag-Pol precursors that are processed into major structural proteins and enzyme proteins. In addition, Vif, Vpr, Vpu, and Env proteins are synthesized from single-stranded RNAs that have undergone the splicing process at the same time.
Among the HIV regulatory genes, Nef is particularly essential for the replication of HIV genetic material in host cells. Nef of HIV acts as a protein-interaction adapter, cell surface receptor expression, cytoskeletal remodeling, vesicular transport, signal transduction, etc. Regulates numerous activities of host cells.[7] Translation of HIV genetic information is a very important process for the expression of genes that regulate these key functions.
1.8. Assembly
Virus aggregation is a very complex and sophisticated process. First, Gag and Gag-Pol precursors interact between three polypeptides, MA (matrix), CA (capsid), and NC (nucleocapsid), and the Gag polyprotein, which consists of three peptides, SP1, SP2, and p6. is synthesized through In addition, Gag proteins are arranged radially under the membrane of HIV, and the nitrogen-terminal MA moiety of Gag reacts with the membrane, and the carbon-terminal NC moiety and p6 moiety are directed toward the center of the particle.[8] Gag and Gag-Pol precursors are myristoylated with nitrogen as the terminus at the MA moiety and condensed on the corresponding particles, and the HIV Env also gathers in through the action with the MA protein. Finally, two copies of HIV RNA, Vif protein, and host cell factors are aggregated in the particle, forming a spherical HIV particle surrounded by a membrane.
1.9. Budding
The process of release of progeny HIV from infected cells is called 'budding'. The p6 late domain of Gag and the Tsg101 protein of the host cell play an important role in the process of circulating the newly generated HIV to escape. The budding process at the end of this HIV replication process is a target for limiting factors. In particular, BST-2 acts to bind infective mature HIV to the cell surface. However, it is inhibited by the reaction of the Vpu, Nef and Env proteins of HIV.
1.10. Maturation
HIV particles are immature, non-infectious, and released in the form of a thick film of radially enclosed Gag and Gag-Pol precursors. During or immediately after budding, the protease of HIV is activated, and the activated protease decomposes Gag and Gag-Pol precursors into the final component of HIV maturation. As a result, the sequence of proteins is reorganized to form an electron-dense conical inner core, and HIV particles become pathogenic.
Ⅲ. Comparison with evaluation of existing treatments according to life history stages
1. Reverse Transcriptase Inhibition
Nucleoside analogue reverse transtriptase inhibitors (NRTIs) and nonnucleoside analogue reverse transtriptase inhibitors (NNRTIs) are representative HIV treatment drugs. NRTIs include Zidovudine (AZT), Didanosine (ddI), Zalcitabine (ddC), Stavudine (d4T), and Lamivudine (3TC), and NNRTIs include Viramune , Nevirapine), Rescriptor (Delavirdine), and Stocrin (Efavirenz). They inhibit the binding of the enzyme to HIV RNA by binding to the reverse transcriptase during the reverse transcription of HIV in the host cell (1.2), thereby modifying the three-dimensional structure of the enzyme. However, when NRTI and NNRTI are administered alone, although effective initially, HIV acquires resistance to this and the drug loses its efficacy.[9]
1.1. Summary of major molecules and elements in each stage of life history
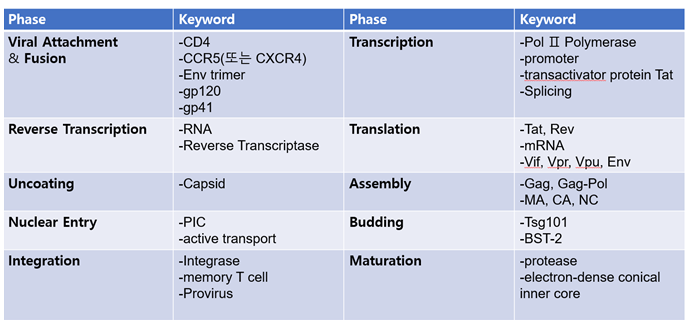
2. Inhibition of proteolytic enzymes
Protease inhibitors (PI) act in the maturation process (1.10) of the life cycle of HIV, that is, in the process of refining the protein molecules of immature HIV. It binds directly to the proteolytic enzyme and alters the enzyme's structure, thereby disrupting its action and allowing the formation of immature, non-infectious HIV particles. Protease inhibitors include Invirase (Saquinavir), Crixivan (Indinavir), Norvir (Norvir, Ritonavir), and Viracept (Viracept, Nelfinavir). However, protease inhibitors also have cross-resistance in which HIV can acquire resistance and are resistant to different protease inhibitors.[10]
3. Other drugs
In addition, there are fusion inhibitors, CCR5 inhibitors, integrase inhibitors, and the like. Fusion inhibitors inhibit the fusion of HIV with the cell membrane of a host cell (1.1), and Nfuvertide corresponds to this. CCR5 inhibitors block the invasion of HIV by interfering with the action of CCR5 receptors in the process of HIV attaching to the surface of host cells (1.1), and maraviroc corresponds to this. The integrase inhibitor inhibits the enzyme that binds to DNA in the process (1.5) when the genetic material of HIV is inserted into the DNA of the host cell, and raltegravil corresponds to this.[11]
4. Side Effects and Limitations
First of all, the currently used HIV, or AIDS treatments, have a variety of symptoms, such as abdominal pain, diarrhea, nausea, vomiting, headache, fever, dizziness, insomnia, anemia, increased cholesterol, changes in body fat distribution, paresthesia in the lower extremities, decreased kidney function, and osteoporosis. may cause side effects.
Also, the treatment used today is that HIV in the body can become resistant to the drug before the treatment is finished. Due to this, the effect of the previously used therapeutic agent disappears and other drugs must be used. However, due to the nature of HIV, which has RNA as its genetic material, mutations occur frequently and resistance to new drugs may be formed.
The most important limitation is that once you start treatment, you cannot stop it. As mentioned in Chapter 1.5, since the genome of HIV has an incubation period in the form of a provirus after it is inserted into the DNA of memory T cells, it not only evades from the body's immune system, but also appears after treatment is stopped and can infect other cells again. there is. Therefore, even when it is found that the number of HIV in the body has decreased or disappeared by administering a therapeutic agent, there is a very high possibility that HIV in the incubation state exists. For this reason, it is very difficult to cure HIV or AIDS at present.
5. HAART
As mentioned earlier, HIV is easily mutated, and in many cases, it acquires resistance to a therapeutic agent, thereby negating its effectiveness. Therefore, cocktail therapy, or potent antiretroviral therapy (HAART), is currently the most effective method of HIV treatment. This refers to a treatment method by simultaneously administering three or more therapeutic agents such as NRTI, NNRTI, and PI. This increases the therapeutic effect by reducing the resistance of HIV. However, HAART also has 6~16% resistance, so there is still a limit.[12]
Ⅴ. conclusion
This study analyzes the life history of the HIV virus, which is the cause of AIDS, which utilizes and disrupts the human immune system, and suggests treatments that can be applied to each stage of life. HIV infects and destroys cells responsible for the immune system, such as helper T cells and macrophages, as hosts. Therefore, since it is impossible to treat with the body's immune system alone, many researchers have developed various treatments through research on HIV treatment for over 30 years. However, in this study, paying attention to the fact that 30 years of research did not find a cure method to completely remove HIV from the patient's body We analyzed and evaluated and compared existing treatments according to life history stages. In addition, the stages of reverse transcription and maturation were presented as key treatment steps that can approach a cure.
In the evaluation and comparison stage of the existing treatment according to the life-history stage, NRTIs and NNRTIs, which are representative reverse transcriptase inhibitors, can be effective in the initial stage when administered alone, but clearly presented the limitation of acquiring resistance to HIV. The risk of cross-resistance to Invirase, Crixivan, Norvir, and Viracept PI substances that can be applied in the maturation stage was analyzed as a limiting point. In addition, side effects and limitations of fusion inhibitors, CCR5 inhibitors, and integrase inhibitors that can be applied in the fusion step were also analyzed.
Finally, resistance to HIV drugs was analyzed as the biggest obstacle to cure. Therefore, we considered the treatment of simultaneous administration of NRTI, NNRTI, and PI, suggesting a 6 to 16% probability of resistance generation in cocktail therapy. Through this study, we tried to devise a larger principle rather than describe the specialized and specific details required for treatment, and to help cure HIV, which has become a chronic disease.
Reference
[1] "코로나바이러스감염증-19(COVID-19) 국외발생현황." 질병관리청, n.d., ncov.mohw.go.kr/bdBoardList_ Real.do?brdId=1&brdGubun=14&ncvContSeq=&contSeq=&board_id=&gubun=. 2022년 2월 9일 접속.
[2] Kirchhoff, Frank. "HIV life cycle: overview." Encyclopedia of AIDS (2013): 4 Fig3. Overview of the viral replication cycle.
[3] In this process, some do not become active for many years after replication, so it is necessary to monitor the status for a long period of time. : Obr, Martin, and Hans-Georg Krausslich. "Viruses: The secrets of the stability of the HIV-1 capsid." Elife 7 (2018): e38895.
[4] Nkeze, Joseph, et al. "Molecular characterization of HIV-1 genome in fission yeast Schizosaccharomyces pombe." Cell & bioscience 5.1 (2015): 1-13. Fig 1. Schematic diagram of HIV-1 genome. The total size of HIV-1 genome is approximately 9.7 kb. Each of the viral genes is drawing based on the relative orientation in the entire RNA genome. Arrows points to cleaved protein products. Dashed lines represent RNA splicing. The number in parenthesis is molecular weight of each protein. LTR long-term repeat, Gag group-specific antigen, MA matrix protein, CA capsid domain, NC nucleocapsid, TF trans-frame protein, Pol polymerases, PR protease, RT reverse transcriptase, IN integrase, Env envelope protein, SU surface membrane protein, TM trans-membrane protein, Vif viral infectivity factor, Vpr viral protein R, Vpu viral protein U, Nef negative regulatory factor, Rev regulator of expression of viral proteins, Tat trans-activator of transcription.
[5] Kirchhoff, Frank. "HIV life cycle: overview." Encyclopedia of AIDS (2013): 3 Fig2. Schematic presentation of the expression of viral proteins that are found in the viral particle and of the mature HIV virion
[6] Abraham, Libin, and Oliver T. Fackler. "HIV-1 Nef: a multifaceted modulator of T cell receptor signaling." Cell communication and signaling 10.1 (2012): 1-11.
[7] Briggs, J. A. G., et al. "Structure and assembly of immature HIV." Proceedings of the National Academy of Sciences 106.27 (2009): 11090-11095.
[8] 오명돈. "HIV 감염증의 치료." 대한의사협회지 50.4 (2007): 316-323.
[9] "약물백과.", HIV 치료제. 약학정보원, n.d., . 2022년 03월 01일 접속.
'Project > Biology_Study after the project' 카테고리의 다른 글
| Chromatin modification (2) | 2022.03.13 |
|---|---|
| Special transcription factors (2) | 2022.03.09 |
| Transcriptional regulation (2) | 2022.03.09 |
| Transcriptional regulation (6) | 2022.02.27 |




댓글